In Chinese each chemical element has a dedicated character usually created for the purpose see Chemical elements in East. 1 as the universal gravitation constant which is a physics unit so we exclude it in chemistry or it can be 2 the SI abbreviation for Giga 109.
Chemical Equilibrium Definition Examples Diagrams
Their composition structure properties behavior and the changes they undergo during a reaction with other substances.
G meaning chemistry. Delta H is the change in enthalpy. Solid s liquid l gas g or dissolved in water aq. Chemistry definition the science that deals with the composition and properties of substances and various elementary forms of matter.
In the previous equation. 1 kilogram equals 1000 grams or 1 kg 1000 g and 1 megameter equals 1000000 meters or 1 Mm. Gibbs Free Energy refers to the energy in a chemical reaction that can be used to do work.
Element symbols for chemical elements normally consist of one or two letters from the Latin alphabet and are written with the first letter capitalised. 4Li s O 2g 2Li 2 O s The state symbols in brackets show the physical state of the substance at the reaction temperature. You will never find G values in tables only delta G values.
Congener - member of the same group of elements of the periodic table eg iodine and chlorine. The Gibbs Free Energy of a reaction delta G can be calculated through the equation delta G delta H - Tdelta S. Everyone is invited to use our website and chemistry dictionary to help further their knowledge students and teachers alike.
In chemistry delta G refers to the change in Gibbs Free Energy of a reaction. The science of the composition structure properties and reactions of matter especially of atomic and molecular systems. All right enough of the chemistry but there is an important point here and any mathematicians will see it.
The pH scale is a logarithmic scale. G as a capital can have 2 meanings. It stands for Oxidation is loss reduction is gain It refers to the transfer of oxygen hydrogen and electrons during a reduction-oxidation reaction between two molecules.
For example the chemical equation 2H 2 O 2 2H 2 O can be interpreted to mean that for each 2 mol dihydrogen H 2 and 1 mol dioxygen O 2 that react 2 mol of water H 2 O form. Well take a look at the definition of an epimer followed by a close look at some specific examples. When associated with a lower case m it would normally be understood to mean a gigametre which is a length 109 metres.
Thank you for visiting. So delta G is used in calculating the K of your reaction. Chemistry and Matter.
The mole is widely used in chemistry as a convenient way to express amounts of reactants and products of chemical reactions. In the scope of its subject chemistry occupies an intermediate position between physics and biology. G itself without the delta is ill-defined thats what I forgot out.
G means grams when used alone. Gibbs free energy is a measure of the potential for reversible or maximum work that may be done by a system at constant temperature and pressure. Gibbs free energy G is defined as.
If G is positive at equilibrium then we will have lots of reactants at equilibrium meaning Q needs to be smaller less than 1 to approach K. It is sometimes called the central science because it. It is a thermodynamic property that was defined in 1876 by Josiah Willard Gibbs to predict whether a process will occur spontaneously at constant temperature and pressure.
Aq is called aqueous which comes from the Latin word aqua meaning water. So delta G of a reaction is a measurement relative to the G of the reactant level. In other words a pH of 60 is 10 times more acidic than a pH of 70 and a pH of 50 is 100 times more acidic than a pH of 70.
This is mainly used while writing the chemical equation. In chemistry OIL RIG is a mnemonic device to help students remember the difference between oxidation and reduction. Our website is a free online chemistry dictionary containing over 1800 chemistry terms and definitions.
As Q gets smaller ie as we get more reactants the term RT ln Q gets increasingly negative and eventually adding that term to a positive G will make G 0 equilibrium will be. Chemistry synonyms chemistry pronunciation chemistry translation English dictionary definition of chemistry. G means gaseous state aq means aqueous solution.
Perhaps you have already noticed that the base unit kilogram is a combination of a prefix kilo- meaning 1000 and a unit of mass the gram Some prefixes create a multiple of the original unit. Chemical symbols are abbreviations used in chemistry for chemical elements functional groups and chemical compounds. This is of course in equilibrium.
The focal point of this lesson is the concept of epimers in chemistry. Chemistry is the scientific discipline involved with elements and compounds composed of atoms molecules and ions. Chemistry Abbreviations Browse 4601 acronyms and abbreviations related to the Chemistry terminology and jargon.
If you do not know the state of a substance see melting and boiling points. Conjugate - multiple chemistry definitions referring to Bronsted acids and bases a compound formed by combining other compounds or the overlap of p-orbitals across a sigma bond. Conjugate acid - HX a compound differing from a base X by a proton.
Gram Equivalent Concept With Example
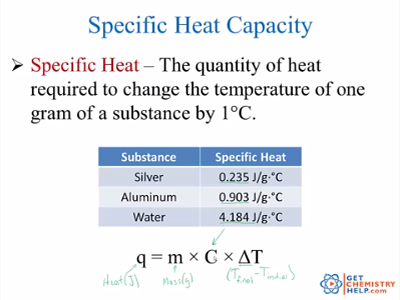
Chemistry Lesson Heat Specific Heat Capacity Get Chemistry Help
Formula Mass The Mass Of An Individual Molecule Or Formula Unit Ppt Download
Introductory Chemistry 3rd Edition Nivaldo Tro Ppt Download
18 8 Gibbs Energy Changes In Chemical Reactions Chemistry Libretexts
8 2 Chemical Equilibrium Chemistry Libretexts
Reaction Stoichiometry Introduction To Chemistry
How To Calculate Percent Yield In Chemistry 15 Steps
13 7 The Gibbs Free Energy Chemistry Libretexts
7 1 The Mole Concept Introductory Chemistry
Free Energy Endergonic Vs Exergonic Reactions Article Khan Academy

0 comments:
Post a Comment